Abstract
Background: Patients with upper extremity deep vein thrombosis (UE-DVT) have unique risk factors determining their incidence and recurrence, and were not included in the large clinical trials leading to drug approval for rivaroxaban and apixaban. There is no published data examining the safety or efficacy of direct oral anticoagulants (DOACs) for UE-DVT.
Objective: Using the Mayo Clinic registry, we evaluated the safety and effectiveness of rivaroxaban and apixaban for the treatment of UE-DVT.
Methods: Consecutive patients treated with rivaroxaban or apixaban for UE-DVT, enrolled into Mayo Clinic Venous Thromboembolism (VTE) Registry between March 1, 2013 and April 30, 2017, were followed prospectively for safety and efficacy. Clinical, demographic and imaging data were collected from each participant at the time of study recruitment. Recurrent VTE, bleeding complications, and death were assessed at 3-month intervals. For subjects unable to return at 3 months, clinical status was assessed by written questionnaire. For unreturned questionnaires, a scripted phone interview was performed. The study was approved by Mayo Clinic Institutional Review Board.
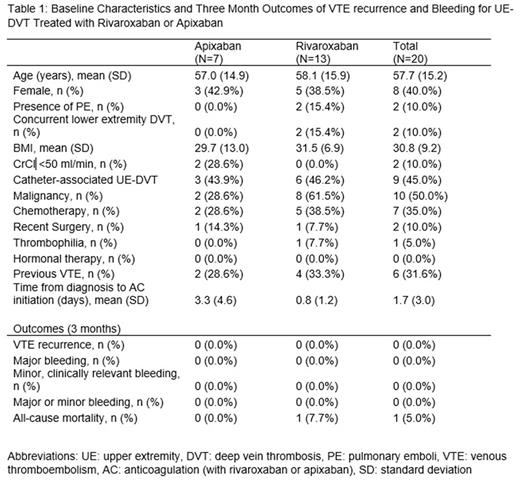
Results: Of the 875 subjects with venous thromboembolism in the registry, 37 had UE-DVT and least 3 months of follow up and 20 were treated with rivaroxaban or apixaban. Three subjects had additional sites of VTE other than UE-DVT (1 with pulmonary embolism, 1 with lower extremity deep vein thrombosis, and one with both). The mean age was 57.7 years (standard deviation [SD] 15.2), mean BMI was 30.8 (SD 9.2), and 12 (60.0%) were male (Table 1). Nine (45%) subjects had catheter-associated UE-DVT, 10 (50.0%) had a diagnosis of malignancy, 7 (35.0%) were receiving chemotherapy, 2 (10.0%) had recent surgery (<6 weeks), and 6 had a prior diagnosis of VTE. The mean time from diagnosis of UEDVT to initiation of treatment with rivaroxaban or apixaban was 1.7 days (SD 3.0). At 3 months of follow up, no subjects had recurrent VTE and no subjects had major or minor bleeding episodes. One patient died from underlying malignancy (32 days after starting rivaroxaban).
Conclusion: In our ongoing registry of subjects with VTE at Mayo Clinic, treatment of UE-DVT with rivaroxaban or apixaban appears to be safe and effective. Further research and a larger sample size are needed to determine the safety and effectiveness of DOACs for UE-DVT relative to other anticoagulants and lower extremity DVT.
McBane: Bristol Myers Squibb: Other: Research grant for cancer associated VTE.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal